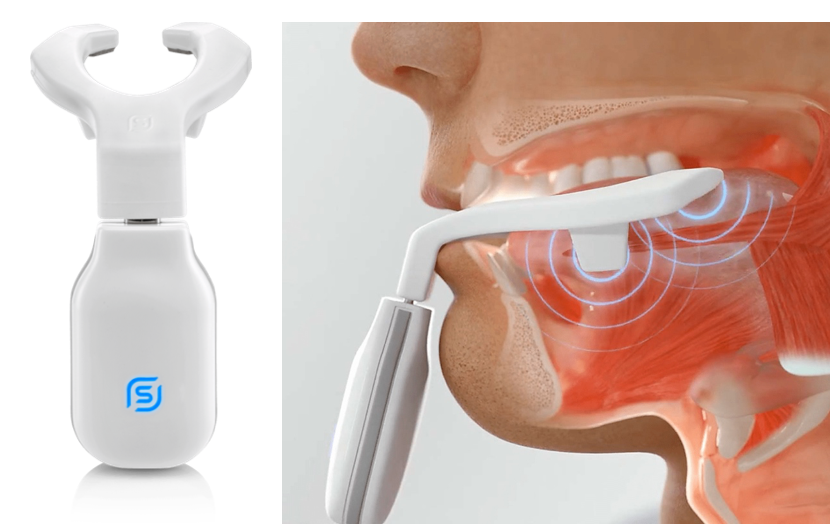
Currently 60 million people in the U.S. suffer from sleep apnea and primary snoring. Most people with OSA snore loudly and frequently, with periods of silence when airflow is reduced or blocked. They then make choking, snorting or gasping sounds when their airway reopens. Needless to say, such irregular, interrupted sleep and accompanying load snoring is detrimental to the health of the sufferer as well as with their partner.
Prevalence
- OSA can occur in any age group, but prevalence increases between middle and older age.
- OSA with resulting daytime sleepiness occurs in at least four percent of men and two percent of women.
- About 24 percent of men and nine percent of women have the breathing symptoms of OSA with or without daytime sleepiness.
- About 80 percent to 90 percent of adults with OSA remain undiagnosed.
- There are few treatment options for mild OSA with snoring
Types
A common measurement of sleep apnea is the apnea-hypopnea index (AHI). This is an average that represents the combined number of apneas and hypopneas that occur per hour of sleep.
Mild OSA: AHI of 5-15
- Involuntary sleepiness during activities that require little attention, such as watching TV or reading
Moderate OSA: AHI of 15-30
- Involuntary sleepiness during activities that require some attention, such as meetings or presentations
Severe OSA: AHI of more than 30
- Involuntary sleepiness during activities that require more active attention, such as talking or driving
Effects of OSA
The health consequences of OSA include increased risk for cardiovascular disease, stroke, metabolic syndrome, reduced quality of life, and premature death[1], resulting from:
- Fluctuating oxygen levels
- Increased heart rate
- Chronic elevation in daytime blood pressure
- Increased risk of stroke
- Higher rate of death due to heart disease
- Impaired glucose tolerance and insulin resistance
- •Impaired concentration
- Mood changes
- Increased risk of being involved in a deadly motor vehicle accident
- Disturbed sleep of the bed partner
Economic Impact
Aside from the health consequences, the economic impact of OSA Several years ago, the American Academy of Sleep Medicine commissioned a white paper that estimated total societal-level costs of OSA to exceed $150 billion per year in the United States alone1. Today, the economic burden of OSA is estimated at close to $170 Billion annually.
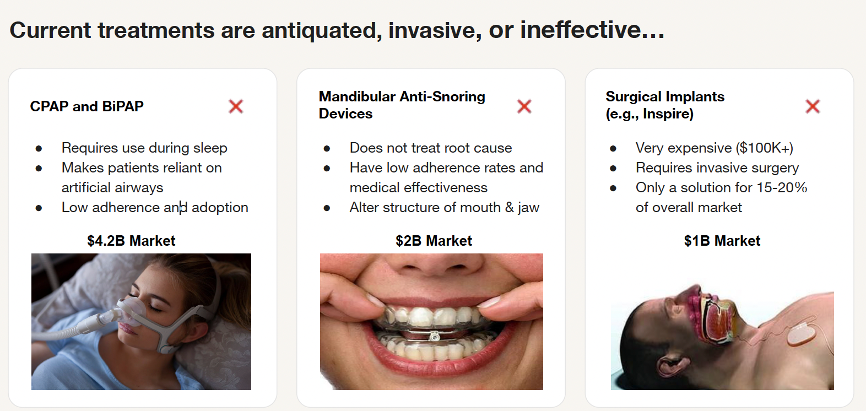
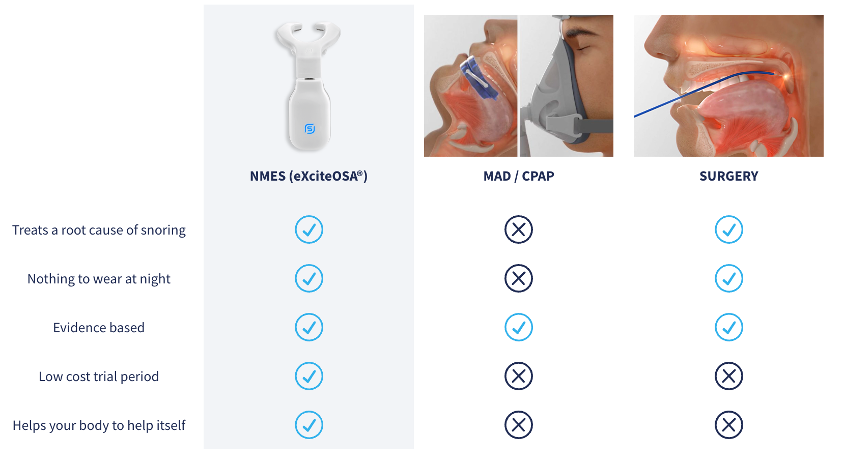
Continuous Positive Airway Pressure (CPAP) and Bi-Level Positive Airway Pressure (BiPAP)
CPAP is the gold standard treatment for patients with symptomatic obstructive sleep apnea. CPAP has few major side-effects, and for most patients an initial trial with CPAP is recommended. Some patients have transformative benefits from CPAP, but new therapies or improvements in existing therapies for obstructive sleep apnea are needed in view of the large number of patients who are intolerant of CPAP or avoid a diagnosis of obstructive sleep apnea because of their concerns about therapy.
The rate of CPAP adherence remains persistently low over twenty years’ worth of reported data. No clinically significant improvement in CPAP adherence was seen even in recent years despite efforts toward behavioral intervention and patient coaching. This low rate of adherence is problematic and calls into question the concept of CPAP as gold-standard of therapy for OSA.
Mandibular Anti-Snoring Devices
Mandibular Advancement Devices (MADs), or anti-snoring mouthpieces, can be effective for mild to moderate snoring and sleep apnea, but they have potential drawbacks, including jaw discomfort, tooth movement, and TMJ issues, especially with long-term use.
Surgically Implanted Devices (Inspire)
Inspire is an FDA-approved implantable upper airway stimulation device that functions like a pacemaker and stabilizes a patient's throat during sleep in order to prevent obstruction. The device consists of three components: a programmable neuro-stimulator located in a chest pocket, a pressure sensing lead that detects patient's breathing, and a stimulator lead that delivers mild stimulation to the tongue nerve. The stimulator is controlled via the patient’s handheld remote control. Patients turn on the device before going to bed to gently stimulate the throat muscles while sleeping. Studies show Inspire therapy can significantly reduce sleep apnea events, improve sleep quality, and reduce daytime sleepiness. The procedure is generally outpatient surgery, and patients can go home the same day. The device requires surgery to implant, which carries inherent risks like infection, pain, swelling, and nerve damage. Some patients may experience temporary tongue weakness, soreness, or other discomforts. Inspire is primarily designed for moderate to severe OSA and may not be effective for all types of sleep apnea or for those with certain medical conditions. The initial cost of the surgery and device, as well as potential follow-up procedures, can be significant.
The device requires battery replacement after a certain period, which involves another surgical procedure.
[1] Wickwire EM. Value-based sleep and breathing: health economic aspects of obstructive sleep apnea. Fac Rev. 2021 Apr 19;10:40. doi: 10.12703/r/10-40. PMID: 34046644; PMCID: PMC8130410.
[2] Rotenberg BW, Murariu D, Pang KP. Trends in CPAP adherence over twenty years of data collection: a flattened curve. J Otolaryngol Head Neck Surg. 2016 Aug 19;45(1):43. doi: 10.1186/s40463-016-0156-0. PMID: 27542595; PMCID: PMC4992257.
Spring Sleep has achieved Medicare/Medicaid approval for reimbursement